Neuroendoscopy Devices Market Advances with Focus on Minimally Invasive Brain Surgery
Neuroendoscopy Devices Market Advances with Focus on Minimally Invasive Brain Surgery
Blog Article
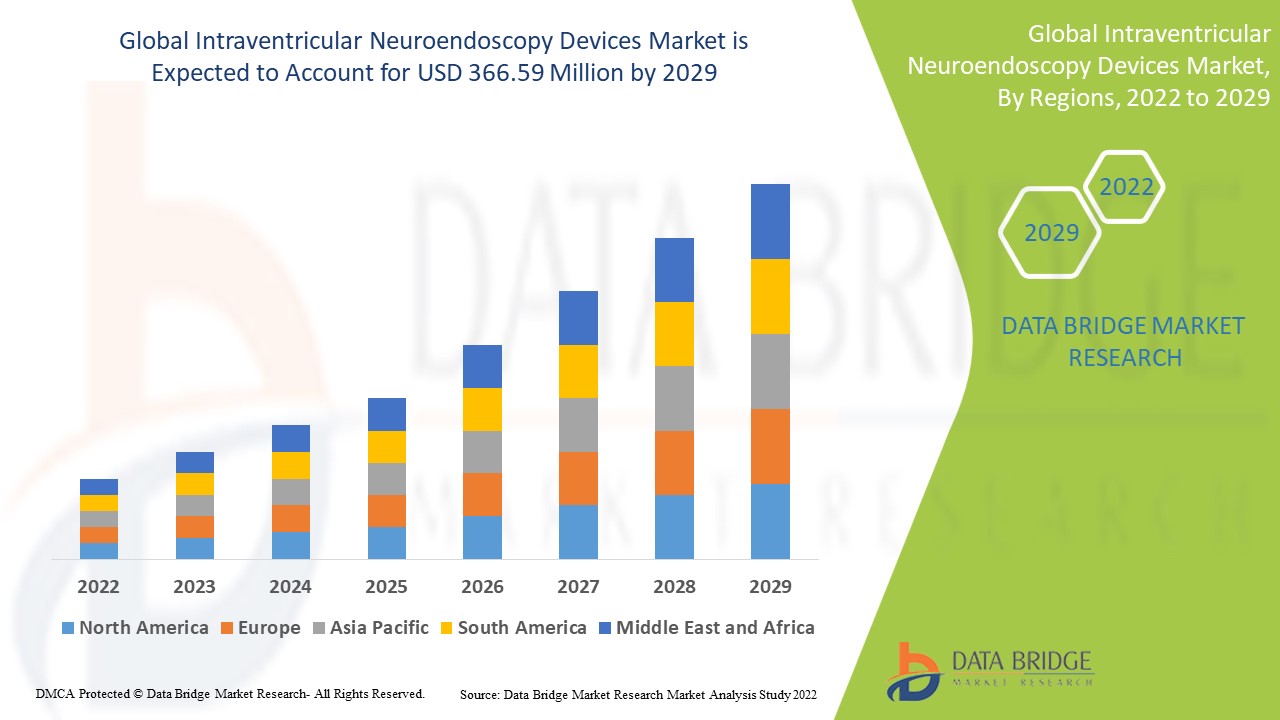
"Intraventricular Neuroendoscopy Devices Market Size And Forecast by 2031
Data Bridge Market Research analyses that the Global Intraventricular Neuroendoscopy Devices Market which was USD 230 Million in 2021 is expected to reach USD 366.59 Million by 2029 and is expected to undergo a CAGR of 6.00% during the forecast period of 2021 to 2029
Intraventricular Neuroendoscopy Devices Market research report provides a comprehensive analysis of the market. The report aims to provide insights into Intraventricular Neuroendoscopy Devices Market trends, growth opportunities, key drivers and challenges, competitive landscape, and other crucial factors that may impact the market in the forecast period (2024-2031).
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-intraventricular-neuroendoscopy-devices-market
Which are the top companies operating in the Intraventricular Neuroendoscopy Devices Market?
The study report on the Global Intraventricular Neuroendoscopy Devices Market offers a comprehensive analysis of the industry, highlighting key trends, market dynamics, and competitive landscape. It profiles prominent organizations operating in the market, examining their successful strategies and market share contributions. This Intraventricular Neuroendoscopy Devices Market report provides the information of the Top 10 Companies in Intraventricular Neuroendoscopy Devices Market in the market their business strategy, financial situation etc.
**Segments**
- **Product Type**: The global intraventricular neuroendoscopy devices market can be segmented based on product type, including rigid neuroendoscopes, flexible neuroendoscopes, and semi-rigid neuroendoscopes. Rigid neuroendoscopes are commonly used in neurosurgical procedures for their precise visualization capabilities. Flexible neuroendoscopes are preferred for their ability to navigate through complex anatomical structures, enabling minimally invasive surgeries. Semi-rigid neuroendoscopes offer a balance between rigidity and flexibility, making them versatile for various neuroendoscopic procedures.
- **Application**: The market can also be segmented by application, with key categories such as intraventricular tumor biopsy, hydrocephalus management, intraventricular hemorrhage treatment, and others. Intraventricular tumor biopsy procedures require high-quality imaging and precision instruments for accurate tissue sampling. Hydrocephalus management involves the effective drainage of cerebrospinal fluid to relieve intracranial pressure. Intraventricular hemorrhage treatment aims to remove blood clots from the brain's ventricular system to prevent further neurological damage.
- **End User**: End-user segmentation in the intraventricular neuroendoscopy devices market includes hospitals, ambulatory surgical centers, and specialty clinics. Hospitals are the primary end users of neuroendoscopy devices due to their extensive neurosurgical departments and advanced infrastructure. Ambulatory surgical centers are gaining popularity for their cost-effective outpatient procedures, while specialty clinics cater to specific neurosurgical needs with specialized equipment and expertise.
**Market Players**
- **KARL STORZ SE & Co. KG**: A leading player in the intraventricular neuroendoscopy devices market, KARL STORZ offers a wide range of neuroendoscopes with advanced imaging technologies and ergonomic designs. The company focuses on innovation and product development to meet the evolving needs of neurosurgeons worldwide.
- **SCHÖLLY FiberoKARL STORZ SE & Co. KG and SCHÖLLY are prominent market players in the intraventricular neuroendoscopy devices market, each contributing significantly to the advancement of neurosurgical procedures. KARL STORZ stands out for its extensive product portfolio that includes rigid, flexible, and semi-rigid neuroendoscopes tailored to meet varying surgical requirements. Their focus on technological innovation and ergonomic design ensures that neurosurgeons have access to cutting-edge instruments that enhance visualization and precision during intraventricular procedures.
Similarly, SCHÖLLY Fiberoptics is known for its expertise in fiberoptic technology, offering high-quality neuroendoscopy devices that provide superior image clarity and flexibility for navigating intricate anatomical structures. The company's commitment to research and development has led to the creation of advanced neuroendoscopes that improve surgical outcomes and patient safety. SCHÖLLY's emphasis on product durability and user-friendly features has garnered a loyal customer base among neurosurgeons seeking reliable tools for intraventricular interventions.
In the competitive landscape of the intraventricular neuroendoscopy devices market, both KARL STORZ and SCHÖLLY emphasize the importance of collaboration with healthcare professionals to gather insights that drive continuous product enhancements. By engaging with neurosurgeons and incorporating their feedback into product development processes, these market players ensure that their neuroendoscopes align with the evolving needs of the medical community. This customer-centric approach not only strengthens brand loyalty but also fosters innovation in intraventricular neuroendoscopy technology.
The market for intraventricular neuroendoscopy devices is witnessing steady growth due to the rising prevalence of neurological disorders requiring surgical intervention. Technological advancements in imaging modalities, such as high-definition cameras and fiber optics, have revolutionized neuroendoscopic procedures by enhancing visualization and precision. As a result, neurosurgeons can perform minimally invasive surgeries with greater accuracy, leading to**Market Players:**
- B. Braun Medical Inc. (U.S.)
- Karl Storz (Germany)
- Olympus Corporation (Japan)
- Zeiss International (U.S.)
- Stryker (U.S.)
- Medtronic (U.S.)
- Ackermann Germany
- Schindlerendoskopie technologie GmbH (Germany)
- Tonglu WANHE Medical Instrument Co Ltd (China)
The market for intraventricular neuroendoscopy devices is experiencing significant growth driven by the increasing incidence of neurological conditions necessitating surgical interventions. Advanced imaging technologies like high-definition cameras and fiber optics have transformed neuroendoscopic procedures by enhancing visualization and precision, allowing neurosurgeons to perform minimally invasive surgeries with improved accuracy and outcomes. This technological progress has led to a surge in demand for innovative neuroendoscopy devices that can address complex neurological challenges effectively and efficiently.
Market players such as B. Braun Medical Inc., Karl Storz, Olympus Corporation, Zeiss International, Stryker, Medtronic, Ackermann, Schindlerendoskopie technologie GmbH, and Tonglu WANHE Medical Instrument Co Ltd are at the forefront of driving advancements in intraventricular neuroendoscopy technology. These companies leverage their expertise in medical devices and surgical equipment to develop cutting-edge neuroendoscopes that cater to the evolving needs of neurosurgeons and healthcare facilities worldwide. By focusing on research and development, product innovation, and customer collaboration, these market
Explore Further Details about This Research Intraventricular Neuroendoscopy Devices Market Report https://www.databridgemarketresearch.com/reports/global-intraventricular-neuroendoscopy-devices-market
Regional Analysis For Intraventricular Neuroendoscopy Devices Market
North America (the United States, copyright, and Mexico)
Europe (Germany, France, UK, Russia, and Italy)
Asia-Pacific (China, Japan, Korea, India, and Southeast Asia)
South America (Brazil, Argentina, Colombia, etc.)
The Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria, and South Africa)
Why B2B Companies Worldwide Rely on us to Grow and Sustain Revenues:
- Get a clear understanding of the Intraventricular Neuroendoscopy Devices Market, how it operates, and the various stages of the value chain.
- Understand the current market situation and future growth potential of the Intraventricular Neuroendoscopy Devices Market throughout the forecast period.
- Strategize marketing, market-entry, market expansion, and other business plans by understanding factors influencing growth in the market and purchase decisions of buyers.
- Understand your competitors’ business structures, strategies, and prospects, and respond accordingly.
- Make more informed business decisions with the help of insightful primary and secondary research sources.
This report provides Global Intraventricular Neuroendoscopy Devices Market :
- An in-depth overview of the global market for
- Intraventricular Neuroendoscopy Devices Market Assessment of the global industry trends, historical data from 2015, projections for the coming years, and anticipation of compound annual growth rates (CAGRs) by the end of the forecast period.
- Discoveries of new market prospects and targeted marketing methodologies for Global Intraventricular Neuroendoscopy Devices Market
- Discussion of R&D, and the demand for new products launches and applications.
- Wide-ranging company profiles of leading participants in the industry.
- The composition of the market, in terms of dynamic molecule types and targets, underlining the major industry resources and players.
- The growth in patient epidemiology and market revenue for the market globally and across the key players and Intraventricular Neuroendoscopy Devices Market segments.
- Study the market in terms of generic and premium product revenue.
- Determine commercial opportunities in the market sales scenario by analyzing trends in authorizing and co-development deals.
Understanding market trends and industry insights at a regional level is essential for effective decision-making. Our reports are available in multiple regional languages to cater to diverse audiences. These localized reports provide in-depth analyses tailored to specific regions, ensuring businesses and stakeholders can access accurate and relevant information. By offering insights in local languages, we aim to bridge communication gaps and empower regional markets with the knowledge they need to grow and thrive. Explore our reports in your preferred language for a more personalized understanding of industry dynamics.
Japanese : https://www.databridgemarketresearch.com/jp/reports/global-intraventricular-neuroendoscopy-devices-market
Chinese : https://www.databridgemarketresearch.com/zh/reports/global-intraventricular-neuroendoscopy-devices-market
Arabic : https://www.databridgemarketresearch.com/ar/reports/global-intraventricular-neuroendoscopy-devices-market
Portuguese : https://www.databridgemarketresearch.com/pt/reports/global-intraventricular-neuroendoscopy-devices-market
German : https://www.databridgemarketresearch.com/de/reports/global-intraventricular-neuroendoscopy-devices-market
French : https://www.databridgemarketresearch.com/fr/reports/global-intraventricular-neuroendoscopy-devices-market
Spanish : https://www.databridgemarketresearch.com/es/reports/global-intraventricular-neuroendoscopy-devices-market
Korean : https://www.databridgemarketresearch.com/ko/reports/global-intraventricular-neuroendoscopy-devices-market
Russian : https://www.databridgemarketresearch.com/ru/reports/global-intraventricular-neuroendoscopy-devices-market
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1530
Email:- [email protected]" Report this page